There are presently approximately some 1,200 different commercial dyes manufactured in the United States, and another 800 are imported. Dyes can be classified on the basis of classification as acid dyes, basic dyes, direct dyes, disperse dyes, fluorescent briighlighters, reactive dyes, sulfur dyes and vat dyes. Dyes are produced by a variety of chemical reactions from raw materials; most of these materials are hazardous to humans. our present studies based on the synthesis of direct blue dye and evaluation of their properties on lab scale.
Direct Dye
An anionic dye used for dyeing cellulosic which required the presence of electrolytes in the dyebath. Direct dyes, a class of dyes largely for dyeing cotton, is water-soluble and can be applied directly to the fibre from an aqueous solution. The term direct dye application stems from some dyestuff having to be either fermented as in the case of some natural dye or chemically reduced as in the case of synthetic Vat and Sulphur dyes before being applied. This renders the dye soluble so that the fibre can absorb it; the insoluble dye has very little substantively to the fibre. Most other classes of synthetic dye, other than vat and sulfur dyes are also applied in this way.
These are soluble in water and have direct affinity for all cellulose fibres. Some will also dye silk and wool. By continuous research this group of dyes has been supplemented with dyes of good fastness to light and washing. As these dyes, when dyed without additives, do not exhaust well, an addition of salt is required to improve the yield of the dye and obtain deeper shades. Generally, the wash fastness of these dyes is inferior but there are a number of after treatments available to improve the wash fastness of the dyeing. Most direct dyes can be stripped of the use of stripping salts (Sodium Hydrosulphite) without harmful effects on the fibres.
Use
Direct dyes dye all cellulosic fibres, including viscose rayon, and most of them also dye wool and silk. They do not dye acetate rayon and synthetic fibres. Direct dyes can be applied well at low temperatures and are therefore suitable for tie-dyeing and batik work. Generally these dyes are used where high wash fastness is not required.
Characteristics Of Raw Materials
Common raw materials used to produce dyes include cyclic aromatic compounds, such as benzene and naphthalene. In addition to the cyclic aromatics, many aliphatic reagents and inorganic chemicals are also used. These include sulfuric acid and oleum for sulfonation, nitric acid for nitration, chlorine and bromine for halogenation, caustic soda and caustic potash for fusion and neutralization, and sodium nitrite for diazotization, as well as ammonia, hydrochloric acid, chlorosulfonic acid, and sodium carbonate, bicarbonate, and sulfide. The heavy metals (copper, chromium, mercury, nickel, and zinc) which are used as catalysts and complexing agents for the synthesis of dyes and dye intermediates are considered priority pollutants. The raw materials commonly brought into the facility in large quantities and stored at the facility in tanks, drums or other containers. The final products are also stored at the facility until shipment to suppliers or end users.
Types Of Different Blue Direct Dyes With Different Shades
The discovery by Sir William Perkin, an English chemist in 1936 that a mauve colouring matter capable of dyeing silk could be prepared by the oxidation of aniline started a vast chain of events. It resulted in production of approximately 8,000 distinctly different dyestuffs being manufactured all over the world and sold fewer than 40,000 trade names. Blue Direct dyes for cellulosics possess excellent light fastness properties. Certain dyestuffs within this range are suitable for high-temperature dyeing of cellulosic/polyester blends. Direct dyes possess excellent levelling properties, are highly tinctoral, have fair to good light fastness, and are economical.
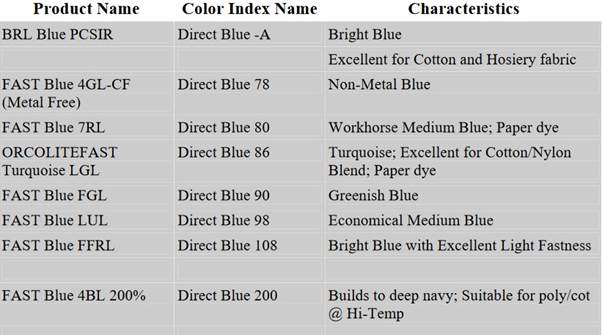
We also have full shade-colour matching programme, textile processing procedure development, and technical problem solving. There are so many branded names of direct blue dye are available in the Table.
The Dyeing Process
Direct dyes are applied to cellulosic fibres from aqueous to which is added an electrolytes, which is usually sodium chloride. The addition of the electrolytes to the dye liquor is essential to obtain adequate exhaustion of the dye molecule by the polymer by the fibres polymer system. When the sodium chloride dissociates completely into the sodium ion and chloride ions. The cellulosic fibres in the dye liquor negative surface charge attracting to sodium cation. The surface of fabric already has a negative and chloride ion both are reple to each other and neutralizes the negative surface charge of the fibre, also referred to as zeta potential enabling the dye anion molecule of the direct dye to enter the fibre more easily and dyed the fabric with help of this phenomena. Heat is also proved to increase the energy of dye molecule to perform the dyeing process very easily.
Dyes And Chemicals
Carient BRL Direct Blue dye, PCSIR Dircet Blue Dye colorants are used for the study and comparison purpose. The other chemicals and auxiliaries added are levelling agent, wetting agent, salts such as sodium sulphate, ammonium chloride, dicyandiamide, urea, copper sulphate and sodium benzoate are AR Grade chemicals.
Equipment:
Dyeing was carried out by using laboratory scale equipment's such as Dyeing bath made by PCSIR, Light fastness tester (MBTL) was carried out fading, Per spirometer was used for shade depth in acidic and alkaline media and spectrophotometer to analysis the data of direct blue dye.
Experimental Work
For the determination of shade depth of direct dye to prepare 1% aqueous solution of dye. Dyeing 1% depth was carried out on 5gm of fabric by keeping dyeing machine at different temperature range 40-1000C and the liquor ratio 1:20.after 5min intervals to check the shade depth of dye remaining solution for the continuous time for 55min at pH =7.5 Absorbance of the diluted liquor after 5min intervals were determine at 462nm.To check the fastness property of direct blue dye with help of standard method of ISO-105.Assess the staining and change in shades to the rating of Grey.
Recipe of Direct Dyeing:
Dye stock solution = 1gm/l00ml
Sodium Chloride = 1gm /l00ml
Conditions for Direct Dyeing
Substrate: scoured and bleached optical brightener free 100% woven fabric (150g/m2 )
pH = 7.5
Drying Temperature = 850C
Dyeing time = 40min
Standard Practice For Spectrophotometer & Description of Color by Using
(1) Munsell Color
(2) CIE System
To determine the precise properties of color. This practice is concerned with the spectral characteristic of light transmitting materials. The wavelength, Hue, Chromatic behavior and Trimulus Value of Direct dyes like Blue –H-acid dye was calculated. The color difference between Blue –H-acid dye was determined by CIE Lab System.
The Munsell Color System.
Albert Henry Munsell, an American artist, devised one of the most influential color-modeling systems. Munsell desired to create a "rational way to describe color" that would use clear decimal notation instead of a lot of color names that he considered "foolish" and "misleading." His system, which he began in 1898 with the creation of his color sphere, or tree, saw its full expression with his publication, A Color Notation, in 1905. This work has been reprinted several times and is still a standard for Colorimetry (the measuring of color).
Munsell modeled his system as an orb around whose equator runs a band of colors. The axis of the orb is a scale of neutral gray values with white as the North Pole and black as the South Pole. Extending horizontally from the axis at each gray value is a gradation of color progressing from neutral gray to full saturation. With these three defining aspects, any of thousands of colors could be fully described. Munsell named these aspects, or qualities, Hue, Value, and Chroma.
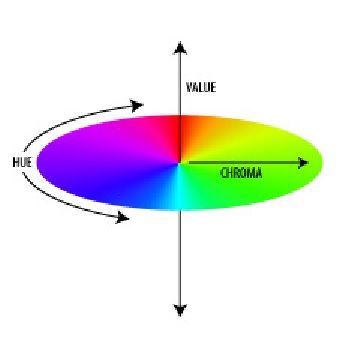
Hue
Munsell defined hue as "the quality by which we distinguish one color from another." He selected five principle colors: red, yellow, green, blue, and purple; and five intermediate colors: yellow-red, green-yellow, blue-green, purple-blue, and red-purple; and he arranged these in a wheel measured off in 100 compass points:
The colors were simply identified as R for red, YR for red-yellow, Y for yellow, etc. Each primary and intermediate color was allotted ten degrees around the compass and then further identified by its place in the segment. For example, primary red would be identified as 5R since it stands at the mid-point of the red segment. 2.5R would be a red tending more toward red-purple, while 7.5R is a red tending more toward yellow red.
Munsell's arrangement of colors in this way was also important for his concept of color harmony, or balance. Munsell was a conservative artist with strict views on the aesthetics of painting. He wanted his system to serve not only as guide for notating colors, but also as a guide for choosing complimentary colors for artistic work.
Value
Value was defined by Munsell defined value as "the quality by which we distinguish a light color from a dark one." Value is a neutral axis that refers to the grey level of the color. This
ranges from white to black. As notations such as 10R, 5YR, 7.5PB, etc. denote particular hues, the notation N is used to denote the gray value at any point on the axis. Thus a value of 5N would denote middle gray, 2N a dark gray, and 7N a light gray. In Munsell's original system, values 1N and 9N are, respectively, black and white, though this was later expanded to values of 0 (black) through 10 (white).
The value of a particular hue would be noted with the value after the hue designation. For example, 5PB 6/ indicates a middle purple blue at the value level of 6. It should be noted, too, that Munsell's scale of value is visual, or perceptual. That is, it's based on how we see differences in relative light, not on a strict set of mathematical values from a light source or illuminant.
Chroma
Chroma is the quality that distinguishes the difference from a pure hue to a gray shade. The chroma axis extends from the value axis at a right angle and the amount of chroma is noted after the value designation. Thus 7.5YR 7/12 indicates a yellow-red hue tending toward yellow with a value of 7 and a chroma of 12:
However, chroma is not uniform for every hue at every value. Munsell saw that full chroma for individual hues might be achieved at very different places in the color sphere. For example, the fullest chroma for hue 5RP (red purple) is achieved at 5/26: Another color such as 10YR (yellowish yellow, red) has a much shorter chroma axis and reaches fullest chroma at 7/10 and 6/10:
In the Munsell System, reds, blues, and purples tend to be stronger hues that average higher chroma values at full saturation, while yellows and greens are weaker hues that average fullest chroma saturation relatively close to the neutral axis. And, reds, blues, and purples reach fullest saturation at midlevels on the value scale, while yellows and greens reach it at higher values (7/- or 8/-).
The result of these differences is that what Munsell originally envisioned as a sphere or orb is radically asymmetrical. A three-dimensional solid representation of Munsell's system. This gave rise to the alternate describing the solid representation as a tree. Munsell's system, although dating back to the 19th century and devised more by intuition than exact science, is still an internationally accepted, leading color system. The Munsell Book of Colors is sold commercially to printers and designers, as are a number of other Munsell color products.
Also available are digital color libraries for Munsell Book of Colors and Munsell High Chroma Colors.
Observation:
The Munsell Colour characteristics of Direct Blue Dye
Chroma is 10. 68,
Hue is 5PB means Purple Bluer Value is 3.17
(2) CIE System:
CIELAB is the second of two systems adopted by CIE in 1976 as models that better showed uniform color spacing in their values. CIELAB is an opponent color system based on the earlier (1942) system of Richard Hunter called L, a, b. Color opposition correlates with discoveries in the mid-1960s that somewhere between the optical nerve and the brain, retinal color stimuli are translated into distinctions between light and dark, red and green, and blue and yellow. CIELAB indicates these values with three axes: L*, a*, and b*. (The full nomenclature is 1976 CIE L*a*b* Space.)
The central vertical axis represents lightness (signified as L*) whose values run from 0(black) to 100 (white). This scale is closely related to Munsell's value axis except that the value of each step is much greater. This is the same lightness valuation used in CIELUV.
The color axes are based on the fact that a color can't be red and green, or both blue and yellow, because these colors oppose each other. On each axis the values run from positive to negative. On the a-a' axis, positive values indicate amounts of red while negative values indicate amounts of green. On the b-b' axis, yellow is positive and blue is negative. For both axes, zero is neutral gray:
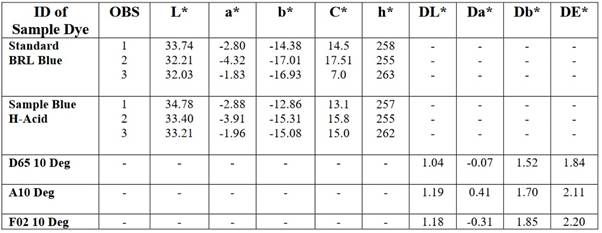
Therefore, values are only needed for two color axes and for the lightness or grayscale axis (L*), which is separate (unlike in RGB, CMY or XYZ where lightness depends on relative amounts of the three-color channels). CIELAB has become very important for desktop color. Like all CIE models, it is device independent (unlike RGB and CMYK), is the basic color model in Adobe PostScript (level 2 and level 3), and is used for color management as the device independent model of the ICC (International Color Consortium) device profiles. Data of CIE L*a*b* for Direct Blue Dye.
Various factors are involved on dyeing process of Direct blue Dyes:
Specific details concerning sampling, weights, volumes, and special techniques to cope with solution and measurement must be established individually for each species of sample and standard dye. Although several of these problems are minimized simply by good analytical technique, some problems are unique to the spectrophotometer measurement of colorants in solution. A few of the pitfalls encountered in solution coloristic are discussed below.
Temperature effect:
To study the effect of Temperature on dyeing process of Direct Blue Dye. In order to specify the Temperature for dye were observed at 400C to 1000C. But during dyeing process were observed the dark shade depth at 850C with good fixing property of direct dye with cold and hot rinsing method. So, the increasing the effect of temperature also effective on dyeing process of direct Blue dye. The remaining solution of direct dye was measure with help of spectrophotometer to find out the absorbance of dye in liquor.
Time effect:
To study the effect of Time on dyeing process of Direct Blue Dye. In order to specify the Time for dye were observed at 0 to 45min. But during dyeing process were observed the dark shade depth at 40min with good fixing property of direct blue dye. So, the increasing the effect of time also effective on dyeing process of direct Blue dye and also find out the result of direct blue dye with the help of spectrophotometer.
pH effect:
The initial pH of the dyebath will be lower at the end of the dyeing process. The cellulosic fibre is responsible for some of this reduction, while a smaller part is used by the dyestuff as it hydrolysis. The effect of pH, account must be taken of the internal pH of the fibre as well as the external pH of the solution. The internal pH is always lower than external pH of the solution. Since the decomposition reaction is entirely in the external solution, the higher external pH favors decomposition of the dye rather than reaction with the fiber. pH influences primarily the concentration of the cellulosic site on the fibre. It also influences the hydroxyl ion concentration in the bath and in the fibre. Raising the pH value by 1 Deg corresponds to a temperature rise at 85o C; the dyeing rate is best improved by raising the dyeing temperature once a pH of 11-12 is reached. Further increased in pH will reduce the reaction rate as well as the efficiency of fixation. Different types of alkalis, such as caustic soda, soda ash, sodium silicate or a combination of these alkalis, are used in order to attain the required dyeing pH. The choice of alkali usually depends on the nature of dye.
The colour of Direct Blue dyes was not change drastically with pH, and in most instances this behaviour is predictable from chemical structure. To provide reliable coloristic data for products that vary with pH, it is necessary to adjust the pH prior to measurement. Adjustment the pH with help of dropper in the dyebath and note the absorbance of Direct Blue dye by the spectrophotometer.
The best value of pH = 10.0 and absorbance of direct blue dye is 0.322 to show the good
condition of dyeing at 850C for 40min of direct blue dye.
Electrolyte effect:
The addition of electrolyte results in an increase in the rate and extent of exhaustion, increase in dye aggregation and a decrease in diffusion. The electrolyte efficiency increases in the order: NaCl<Na 2 SO4 < NH4 Cl. There are so many other salts to be used, such as Urea, Sodium bonzates, Copper sulphate, and Dicyanodiamide. Copper sulphate is very effective salt to improve the shade depth of direct blue dye during on dyeing processes and improved the wash fastness property of direct blue dye.
Colour Fastness Properties of Direct Blue Dyes:
Colour Fastness to Light (artificial light sources):
Dyed and printed direct colour has a moderate light fastness, the light fastness rating being about-3. this means that the direct dye anions seem to take a stable electron arrangement, particularly in the Chromophore. A relatively short exposure to direct light is enough to initiate the degradation of the dye molecules. The resultant breaks down of direct dye anion is seen as fading of the dyed or printed cellulosic textile material.
Colour Fastness to Washing:
The washing fastness property of direct blur dye is about 2-3. The comparatively poor
washing fastness
Cellulosic material dyed with direct dye can be explained as below.
The relatively large numbers of auxochromes are present in the direct dye anion, which contributes to the aqueous solubility in water. The direct dye are attached to the cellulosic polymer by the hydrogen bonding and Vander wall forces, but both are weak forces in aqueous condition such occur in laundering these weak bonds may be hydrolyzed by the water molecule resulting in the removal of the dye on the polymer system. The loss of direct dyes is seen as light shade appears on the cellulosic material.
Colour Fastness to Perspiration:
The perspiration property of direct blue dye is 2-3 because of the weak bonding and easily chemical degradation of dye in the aqueous solution of water.
Optimum Values
Direct blue dye was prepared and data analysis to achieved good exhaustion dyeing method
as well as fastness properties.
• The optimum value of Temperature is 850C.
• The optimum value of Time is 40min.
• The optimum value of PH is 7.5
1. Colour Fastness Property of Direct Blue Dye
• Colour Fastness to Light: The Blue Wool Scale rating of Fading is 3.0 (moderate
• rating)
• Colour Fastness to Washing: The Grey Scale rating of change in shade is 2.0 - 3.0
• Colour Fastness to Perspiration: The Grey Scale rating of change in shade is 2.0 - 3.0
2. The Munsell Colour of Direct Blue Dye:
To determine the different colour observation of direct blue dye with help of Munsell colour
diagram.
• Chroma is 10. 68,
• Hue is 5PB means Purple Blue and
• Value is 3.17
3. CIE L* a*b* Colour System of Direct dye:
CIE L* are of Direct dyes reading =34.78
33.40,
33.21
CIE a* are of Direct dyes reading =-2.88
-3.91
-1.96
CIE b* are of Direct dyes reading =-12.86
-15.31
-15.08
References:
1. E.R Tortman, Dyeing and Chemical of Textile Fibres Edition (1984)
2. CIE Publ. No.15.2, Colorimetry, Second Edition (1986). (No.15.3 to be published).
3. K.Venkataramen, The Chemistry of Synthetic Dyes Vol.R.McDonald, Acceptability and
perceptibility Decisions using the CMC Colour Difference formula, Textile Chemist and
Colorists 20-6, (19 Academic Press N.Y. (1972)
4. Jour. Chem.Soc.Pak.Vol.27, NO.4, 2005.
5. J Park, JSDC, 107 (1991) 93.
6. R. Stanziola, Col. Res.Appl, 5(1980) 129.
7. D.G.Phillips, Col. Res. Appl., 7(1982) 28.
8. D.H.Alman, Col. Res. Appl, 11 (1986) 153.
9. B.Sluban, Col. Res. Appl., 20 (1995) 226.
10. Standard Method for the Determination of the Colour Fastness of Textile and Leather 5th
Edition Bradford S.D.C. (1991)
11. F J J Clarke, R McDonald and B Rigg, JSDC, 100 (1984) 128,281










Comments